By now, we’ve all heard that the development of a COVID vaccine is moving along at a much faster pace than normal. And this frightens some folks. The catchphrase for this program, Operation Warp Speed, isn’t helping. We envision our vaccine development process hurtling through the uncharted space of a science fiction movie – not so confidence inspiring. This is a misstep that we will hopefully learn from in the future. Words matter. But maybe you’ve also heard the phrase “Necessity is the mother of invention”? Well, despite the unfortunate naming, we can have confidence in this new and improved vaccine development process. Read on and I’ll explain. There’s a COVID19 vaccine coming soon: here’s what you need to know – Part 2.
The typical vaccine development process takes, on average, 10-15 years. So, how is it that we will have a vaccine available in 12-18 months? Is this safe? Aren’t corners being cut to make this happen so fast? The short answers to these last two questions are Yes and No. Yes, it is safe. No, corners are not being cut.
In an effort to more fully understand our future COVID vaccines, we first looked at the function of the immune system and how vaccines work. Today, we’ll tackle normal vaccine development. And we’ll comment on steps where efficiencies have occurred that have allowed this process to proceed more quickly and smoothly for COVID vaccines. Then, we’ll look at vaccine safety monitoring and discuss the existing and additional steps that will be in place to ensure safety of our future COVID vaccine(s).
How are vaccines developed?
Dollars and cents
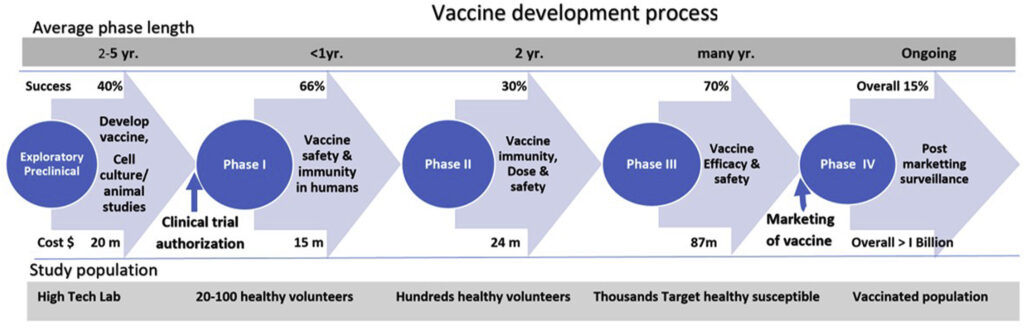
Phases of vaccine development. The cost of developing a vaccine can be prohibitive but during COVID19, scientists have all the money and resources they could ever need. Photo credit Journal of Clinical and Experimental Hepatology.
First, I want to draw your attention to the cost estimates (below the blue arrows) for making a vaccine. It is hugely expensive to make vaccines, typically greater than one billion dollars per successful vaccine. And keep in mind that many vaccine trials aren’t successful, resulting in millions of dollars spent without anything to show for it. There is great potential for financial loss in this endeavor. And finding funding for these projects, traditionally, is one step that can slow the vaccine development process down.
But during COVID, money and staff and resources are being thrown at scientists hand over fist. Some drug companies are footing the bill themselves, betting on a lucrative contract for vaccine distribution at the end. But many labs are being funded by various governments, investing in the hope of a successful vaccine to end this Pandemic.
While we’re on the subject of money… In a typical (pre-COVID) vaccine development process a vaccine would finish it’s human trials and, if successful, then begin the process of mass production. But another thing that the plentiful supply of money is allowing is for drug companies to begin mass production BEFORE the human trials are over.
They are scaling up production to be ready for IMMEDIATE distribution, once the human trials have ended. This is risky for the investors, as not all trials will result in a successful vaccine. But the investors are willing to take that risk, given the devastating effects that COVID is having on our people and economy. We are working against the clock. And money is buying us time AND lives.
Pre-clinical efficiencies

The Exploratory/Pre-Clinical phase typically takes years but here’s where we’ve saved lots of time. Photo credit Journal of Clinical and Experimental Hepatology.
Next, let’s look at the first stage of vaccine development – the Exploratory/Preclinical phase (see first blue circle on the left). There are several things that happened during this phase of COVID vaccine development that moved the process along much more quickly.
- International cooperation – Scientists came up with the genetic sequence for the SARS-CoV-2 virus within a month of the virus’ discovery. And that genetic sequence, required for development of a vaccine, was then shared across the world. This go around, each lab didn’t have to come up with the genetic sequence on its own.
- We had a head start, thanks to SARS and MERS – SARS-CoV-2 is genetically similar these two other coronaviruses, with which we already had experience and for whom vaccines were already under development. Scientists didn’t have to start from scratch. They already had the building blocks of laboratory investigation. From research on SARS and MERS, we knew, for example, which vaccine platforms should work against this virus. And we knew that the spike protein was probably the best target to include in the vaccine. Consequently, the pre-clinical phase, which typically takes years, instead took months.
- Newer technologies – If Pfizer/BioNTech or Moderna are the first to get approval for their COVID vaccines, this will be the first time an mRNA vaccine has ever been used in humans. However, this vaccination platform is not new. It has been used for some years in attempts to develop other vaccines. Those vaccines just haven’t yet come to fruition. One of the wonderful things about this technology is that it is rapidly scaleable (meaning lots and lots of vaccine can be made quickly). With other platforms, it takes a fair amount of time to grow the virus in cells, then to treat the virus such that it loses its infectivity. MRNA does not rely on living cells (in vivo) to grow. It can be transcribed (copied repeatedly and in high volume) in a test tube (in vitro). This means more time saved.
Clinical phase efficiencies
After the pre-clinical phase of vaccine production, human testing in the clinical phases begins.

The redundancies and red tape that have traditionally slowed down Phases 1-3 of human trials have been significantly reduced. Photo credit Journal of Clinical and Experimental Hepatology.
These phases use progressively larger numbers of participants (Phase 1 – 10s, Phase 2 – 100s, and Phase 3 – 1000s) to evaluate vaccine tolerability, immunogenicity (the ability to generate a strong enough immune response), and dosing. And at every step along the way, safety and efficacy are closely monitored. Phases 1 & 2 tend to be fairly redundant, just using increasing numbers of study participants. In recognizing this, the FDA adjusted its requirements for repeat evaluation after each phase (assuming the vaccines were meeting their safety and efficacy goals). This permitted vaccine manufacturers to combine phases of their trials (phase 1/2 or phase 2/3, for example) and to move more seamlessly from one phase to the next, without all the red tape typically required. So, instead of the process running more linearly, each step having to await oversight and approval before moving to the next, the steps of COVID vaccine production have run more in parallel.
Pandemic influences on rapid vaccine production

Turns out, virtual meetings can be a much more efficient way to get things done! Photo credit Pixabay.
In pre-Pandemic times, vaccine oversight committees would have to meet in person – taking days for travel to gather everyone together. However, to limit spread of COVID19, meetings are now required to take place virtually. It turns out that this is a much more efficient use of time and resources. Deliberation of the committees is now much faster.
Also, the fact that the virus is running so rampant through the world, while a bad thing for those it infects, is actually a good thing for speedier vaccine production.
- Faster enrollment – Given that COVID has been so detrimental to our lives and livelihoods, more people have been willing to enroll in vaccine trials. Recruitment has been much faster than normal.
- Lots of virus to go around – Additionally, the virus being targeted has to be prevalent enough in the communities for trial participants to come into contact with it. In order to test how effective a vaccine is, there have to be enough people in the trials who get sick with the virus to tell whether the vaccine was protective or not. If very few trial participants get sick, it will take much longer to get adequate numbers to evaluate safety and efficacy. When lots of people get sick, we can make this determination much more rapidly. Fortunately and unfortunately, low COVID case counts are not something we have had to deal with.
So, there you have it. COVID vaccine production is happening at a much faster clip than has been the case with other vaccines in years past. But this is because of wonderful efficiencies that are occurring in the development process, not because of any corners being cut.
Both before COVID and since, vaccines are the MOST rigorously tested medical interventions that we have. They have to be, because we give them to completely healthy people. The bars for safety and efficacy are set extremely high.
Speaking of safety… Let’s look at how vaccine safety is ensured and monitored.
Vaccine safety monitoring
Safety during vaccine development

Before research can begin, scientists have to apply for IRB approval (see discussion below). Photo credit imarcresearch.com.
There are many stages of safety evaluation that occur DURING vaccine development. Proposed research first has to undergo approval by an Institutional Review Board (IRB). And if there are multiple institutions involved in the research, each institution must give their own IRB approval. The study design must adhere to strict research standards and demonstrate how researchers will protect participants and ensure that ethical standards for human research are followed.
Once the study is underway, safety is first established in animal testing. Only then would a vaccine be moved into human trials. Early phase trials then focus on healthy adults and, only when safety is proven in this group, are more vulnerable populations added to the trials (the elderly, children, people with immune suppression, etc.). And when it comes to children, only after safety is established in older teens and young adults are trials expanded to include younger and younger children. For example, Pfizer and Moderna are only just now adding children over 12 to their studies. Inclusion of even younger children will come at a later date.

The FDA provides oversight for vaccine safety, purity, potency, and efficacy. Photo edited from Pixabay.
The U.S. Food and Drug Administration (the FDA) is charged with monitoring the vaccine development process. Regulations require there to be proof of safety, purity, potency, and effectiveness of all vaccines. If, at any point during testing, the FDA or others detect potential safety signals, trials are put on hold until these signals can be investigated.
This is what happened during the AstraZeneca COVID19 vaccine trial. A vaccine-receiving participant developed serious neurologic symptoms and, until researchers could determine whether or not the condition was related to the vaccine, the trial was put on hold. This is what is supposed to happen. These are the checks and balances for safety that are supposed to exist. I, for one, was happy to see the trial paused. It is proof that the scientists are following protocol – that they are not cutting corners for the sake of rushing a vaccine to market.
Post-marketing vaccine safety monitoring
Very rare adverse events, potentially not seen in studies of thousands or tens of thousands of research participants, may become evident once vaccines are given to millions of people. Consequently, after a vaccine is released to market, the safety monitoring doesn’t end. In fact, there are NUMEROUS robust and broad-ranging systems of surveillance that track vaccine safety for years to come. And when a brand new vaccine is introduced in an emergency use scenario, these systems will be more important than ever.
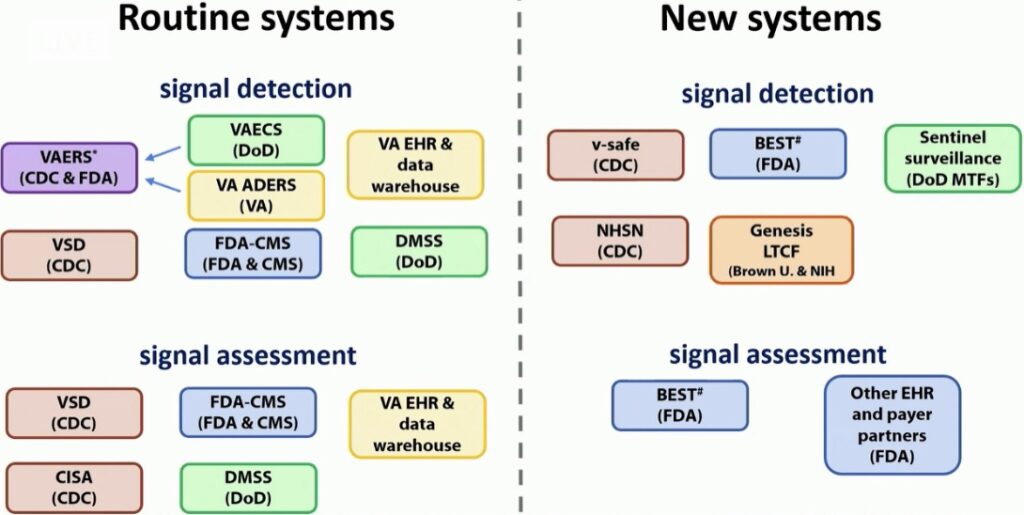
Slide copied from the CDC’s Advisory Committee on Immunization Practices (ACIP) report during recent public meeting on vaccine safety. Photo credit ACIP.
What’s already in place:
- Vaccine manufacturer monitoring –
- Manufacturers may choose to conduct Phase 4 trials. These continue to monitor for safety and efficacy once a vaccine is in use in the broader population.
- Monitoring the general public –
- VAERS – the Vaccine Adverse Events Reporting System – administered by the CDC & FDA. This is a national system that takes reports from, well, anyone about possible adverse events following vaccination. It allows the CDC and FDA to monitor for any signals of problems with a vaccine after its release for use in the general public. If any signal of concern is detected (unexpected events are noted or appear to happen more often than expected or display other unusual patterns), these are then further investigated with specific studies conducted by organizations shown below. Data is analyzed weekly so signals can be detected in near-real time.
- VSD – the Vaccine Safety Datalink – administered by the CDC. The VSD is a collaboration between the CDC and nine different national healthcare organizations across the country. These organizations perform active surveillance and research. They can study any signals detected by VAERS to help determine if adverse events were truly related to vaccination.
- CISA – The Clinical Immunization Safety Assessment project – administered by the CDC. This organization is a collaboration between the CDC and seven research centers. They offer expert consultation on individual cases and also conduct clinical vaccine safety research.
- Medicare data – Administered by the FDA & Centers for Medicare and Medicaid Services (CMS). This is an active surveillance system that allows research on claims-based data (if your doctor submits a claim for “adverse vaccine reaction”, for example).
- BEST – the Biologics Effectiveness and Safety System – administered by the FDA. This is part of the Center for Biologics Evaluation and Research (CBER) surveillance program. It is another claims-based data and electronic health record monitoring system that allows active surveillance and research. It monitors the safety of vaccines and other biologic products (such as blood products, human tissues, and others).
- Sentinel Initiative – Administered by the FDA. Sentinel is another surveillance system using real-world data to study medical product (like vaccines and proposed therapies to treat COVID) safety.
- Monitoring the military –
- DoD VAERS – VAERS for Department of Defense (DoD) populations. See VAERS information above.
- VAECS – the Vaccine Adverse Event Clinical System. This is a system that allows tracking of adverse events following immunization in DoD populations.
- DMSS – the Defense Medical Surveillance System. This is the DoD’s system for active surveillance and research based on electronic health record and administrative data.
- Monitoring for Veterans –
- VA ADERS – the VA Adverse Drug Event Reporting System – administered by the Department of Veterans Affairs. This is a national reporting system to monitor for adverse events following drug or immunization administration in the VA population.
- VA EHR and Active Surveillance System. This system permits surveillance and research based on electronic health record and administrative data.
- Monitoring for Tribal Nations –
- IHS VAERS – VAERS reporting for populations served by the Indian Health Service and Tribal facilities. See VAERS information above.
What’s new since COVID19:
- V-SAFE – Vaccine Safety Assessment for Essential Workers – administered by the CDC
- This represents a new active surveillance system. It will seek out any adverse reactions as opposed to waiting passively for reports to roll in. It will be utilized for early vaccine recipients. Using smartphone and web-based technology, it will provide regular text messages and web-based surveys from the CDC to check in with vaccine recipients for any health problems following vaccination. Any person reporting a clinically important event will then receive telephone follow up and this may then trigger a VAERS report.
- NHSN – National Healthcare Safety Network – administered by the CDC
- NHSH is a pre-existing acute care and long-term care facility (LTCF) infection tracking system. It is well positioned to now also track weekly vaccine doses for healthcare workers and LTCF residents, identify any adverse events among recipients, and report to VAERSs.
- Genesis – a partnership between Brown University and the National Institutes of Health – National Institute on Aging.
- This will serve as a signal detection system for long-term care facility residents.
- Large insurer/payer databases –
- The FDA will use these systems of administrative and claims-based data to conduct surveillance and research.
As you can see, the effort to monitor safety following use of vaccines in the general population is exhaustive (and exhausting – I’m tired just from typing it all out). It is also redundant. And this is a good thing. We want multiple different sets of eyes watching for any signals of concern. This is how checks and balances should work. And they do work!
Nothing in life is risk-free
There is no risk-free option. There is only less risk and more risk. Choosing not to get the COVID vaccine comes with its own potential serious risks. Photo credit Pixabay.
What we need to remember is that nothing in life is 100% risk-free. We can live our lives in a bubble, fearing everything and everyone, or we can accept that there is risk inherent in just living. While there are known potential adverse reactions to vaccines, these typically show up within the first couple of months after immunization – and we will have this data by the time a vaccine for COVID is released. If it is not safe, it will not be approved.
Some will ask, “But what about long-term safety concerns with a COVID vaccine?” And, they are right. We won’t know this for some years to come. But while I struggle to think of any long-term adverse effects from immunizations in current use, I don’t struggle to think that COVID could have far-ranging effects that we will see as years pass. We already see the lingering effects in some who’ve been infected – those suffering from the so-called “Long-Hauler’s Syndrome”. People are struggling with persistent fatigue, brain fog, depression, joint pain, intolerance to exercise or even minimal exertion, and more… months after their initial infection. THIS is what I spend my time worrying about.
There is risk in every decision we make. Choosing NOT to get a COVID vaccine, that has undergone rigorous efficacy and safety testing, seems the riskiest choice of all.
Join me next time for COVID19 vaccine coming soon: here’s what you need to know – Part 3. We will delve into the nitty gritty of the different types of vaccines most likely to see first approval.
PS – since many folks have asked, you can make sure to get each installment in this series by entering your email in the “subscribe” field on my home page. Thanks for following along!


Would love to follow you, thanks for the in reply well written and informative posts.
Thanks, Jennifer. You can find a subscription field on the home page. If you enter your email address then you should receive posts automatically!
Dr. LaSalle, Thank you very much for such clear and informative information. It is so helpful to read a piece that speaks to virtuality all of the questions and concerns that many people have regarding a vaccine for Covid 19. I will be passing your writing along to others as encouragement to get vaccinated as it becomes available to them.
Thank you so much, David!
Hi Dr. LaSalle,
Amazing. You’re clarifying so much for me – and I work in Communications at Spokane Regional Health District!
I wanted to let you know that we rebranded CDC’s “Journey of a Vaccine” and it lives on our web site if you’re interested in having look-see:
https://srhd.org/media/documents/JourneyVaccine.pdf
Keep it up – WE NEED YOU!
Thank you so much for this information, timely and extremely important. May I share it as a guest blog in my website and social media channels? Everyone needs to read this!
Absolutely! Thank you, Shira!