Parts 1, 2, and 3 of this series discussed a variety of topics; the immune system and how vaccines work; the phases of vaccine development and how we have much more rapidly, but safely, developed COVID vaccines; and specifics about the mRNA vaccines. Well, this post is going to be a hodge podge of different topics. There’s a COVID19 vaccine coming soon: here’s what you need to know – Part 4!
I’ll catch up on the answers to some questions that I missed in the last installment of this blogpost. We’ll dive a bit deeper into reported adverse reactions (anaphylaxis, Bell’s palsy, lymphadenopathy). I’ll touch base on the not-entirely-truthful claims that vaccine manufacturers are “liability free”. And we’ll do a head-to-head comparison of the Pfizer and Moderna vaccines. Spoiler alert… they are very similar. So, buckle up. Here we go!
Your questions (take 2)

You, dear readers, had some great questions! Photo credit Pixabay.
As much as I tried in my last blogpost to anticipate questions and concerns, I missed a few things. And you, my astute readers, gave me some really great food for thought. So, let’s look at what you were asking…
1. Can I get my flu shot and the COVID vaccine at the same time?
The COVID vaccine was not studied in conjunction with other vaccines (administered at the same time or in proximity). So, we don’t know how this vaccine, given with others, would affect the response to either one. We give plenty of other vaccines together but we haven’t studied this specifically with COVID vaccines so we can’t recommend doing so… yet. For now, the CDC guidance is:
* Do not give the COVID19 vaccine at the same time as other vaccines
* Do not give the COVID19 vaccine within 14 days of (either before or after) other vaccines
Also, please get your flu shot YESTERDAY! Flu season is upon us (though certainly seeming milder than years past, thanks to all the masking and social distancing and sanitizing). It takes 2 weeks for the flu shot to work so please get it ASAP.
2. Will the vaccine be needed yearly, like the flu shot?
The answer to this is pretty simple. We’re not sure. The coronaviruses as a group are much more stable, less prone to mutation, than the influenza virus. However, we do know that there have been at least a couple of mutations of SARS-CoV-2 so far. Also, immunity following COVID19 infection seems to be relatively short-lived. We have seen people get sick with COVID twice. All together, this would seem to indicate that we will likely need booster vaccines going forward. Whether this is definitively true, and how often we might need said booster shots, will only be learned after we monitor vaccine recipients over time.
3. Will I need a COVID test before getting the vaccine?
The guidelines currently suggest NOT getting a COVID vaccine if you are actively infected with COVID. You should wait until you meet criteria to end your isolation. “But with so many asymptomatic infections, how would I know if I had COVID without testing?”, you may rightly ask. Here is what you need to know about why COVID testing prior to vaccination is NOT recommended:
* There were participants in the vaccine trials who had evidence of prior COVID infection. Safety and efficacy data for those individuals was similar to those who had not had prior COVID infection.
* It would be a logistical nightmare and highly expensive to test everyone before vaccinating. And we know that the nasal swab test can be negative one day and positive the next so this really isn’t practical or helpful.
* FYI, this is the same guidance we give for all vaccines. Essentially, if you have moderate to severe illness, we recommend holding off on vaccination until you have recovered. If something were to go awry after immunization during significant illness, it would be impossible to know if it was the vaccine or a worsening of the underlying illness that caused it.
4. I know we’re still supposed to get the COVID vaccine even if we had COVID. But, what if I received monoclonal antibodies or convalescent plasma to treat my initial COVID illness?
Excellent question! Both of these treatments work by giving the person with COVID illness antibodies against the infection – antibodies that they themselves have not yet made. In the case of monoclonal antibodies, these are developed in the lab. In the case of convalescent plasma, these are taken from a person who has already recovered from COVID and, therefore, has a plentiful supply of antibodies in their blood. But circulating antibodies from monoclonal antibody or convalescent plasma treatment might fight against the vaccine and make it not as effective. So, CDC guidance is as follows:
* Wait 90 days after receiving monoclonal antibodies or convalescent plasma to receive the COVID vaccine
Reported adverse events following the mRNA COVID vaccines

All medical interventions have the possibility of side effects. Are the conditions detected in the COVID vaccine studies (such as Bell’s Palsy and anaphylaxis) REALLY related to the vaccines? Photo credit Pixabay.
We’ll learn more about the Moderna vaccine farther along in this blogpost but noted adverse events following both vaccines were similar. Whether seen in testing of the vaccine (Bell’s palsy, lymphadenopathy) or in real world use of the Pfizer vaccine (anaphylaxis), there are some events noted that are drawing people’s attention and worry. Let’s look at each one and talk about risk.
But first, a reminder about nomenclature (the defining of terms). I specifically use the term “adverse events” instead of “adverse reactions” because they mean two different things. Please keep these in mind as you are reading about vaccines.
Adverse event – In scientific lingo, an adverse event is an event that happens in proximity to some intervention (in this case, vaccination) that may or may not be related to the intervention. An example might be fainting following getting a shot. This could be related to the vaccine but it also may very well NOT be related. Plenty of people faint when getting shots or blood draws due to more emotional/fear reasons than anything.
Adverse reaction – an adverse reaction is a reaction that most likely IS related to the intervention in question. For example, that sore arm you get, that makes you feel like somebody punched you, after receiving a Tetanus shot IS caused by the vaccine.
1. Anaphylaxis
Anaphylaxis is a severe and potentially life-threatening allergic reaction. It most often happens rapidly (within about 30 minutes of exposure) and can result in shock (low blood pressure, rapid heart rate, etc.) and swelling of the mouth, throat, and airways causing difficulty breathing. Some experience nausea, vomiting, or diarrhea and skin reactions (like widespread hives). If recognized, it is highly treatable and almost always recoverable.
People with known anaphylaxis to components of the COVID vaccines were excluded from both the Pfizer and Moderna trials. Additionally, people with an anaphylactic reaction to ANY vaccine(s) were excluded from the Pfizer trial.
* Currently, it is advised that people with known anaphylaxis to components of the COVID vaccines should NOT get this vaccine (see below for vaccine ingredients).
Since administration of the Pfizer COVID vaccine to the first phase of recipients, there have been 6 cases of anaphylaxis noted out of 272,000 doses of vaccine administered in the US. All were treated and have recovered. This is not unexpected. In the Pfizer and Moderna trials, there were no anaphylactic reactions that occurred in proximity to receiving the vaccine. But these studies, together, looked at approximately 74,000 individuals. And only half of these received the vaccine. We might expect to see more cases as these vaccines are administered to hundreds of thousands or millions of people.
Of note, the background rate (rate in the general population) of anaphylaxis is 50-200 episodes per 100,000 person-years with a lifetime prevalence of about 0.05-2.0%. The rate of anaphylaxis in persons receiving the Pfizer vaccine thus far is 0.002% (6 cases out of 272,000 doses administered). This is significantly lower than the background rate of anaphylaxis in the general population.
See below for a workflow for “triaging” (so to speak) people with various allergies when considering the mRNA COVID10 vaccination.
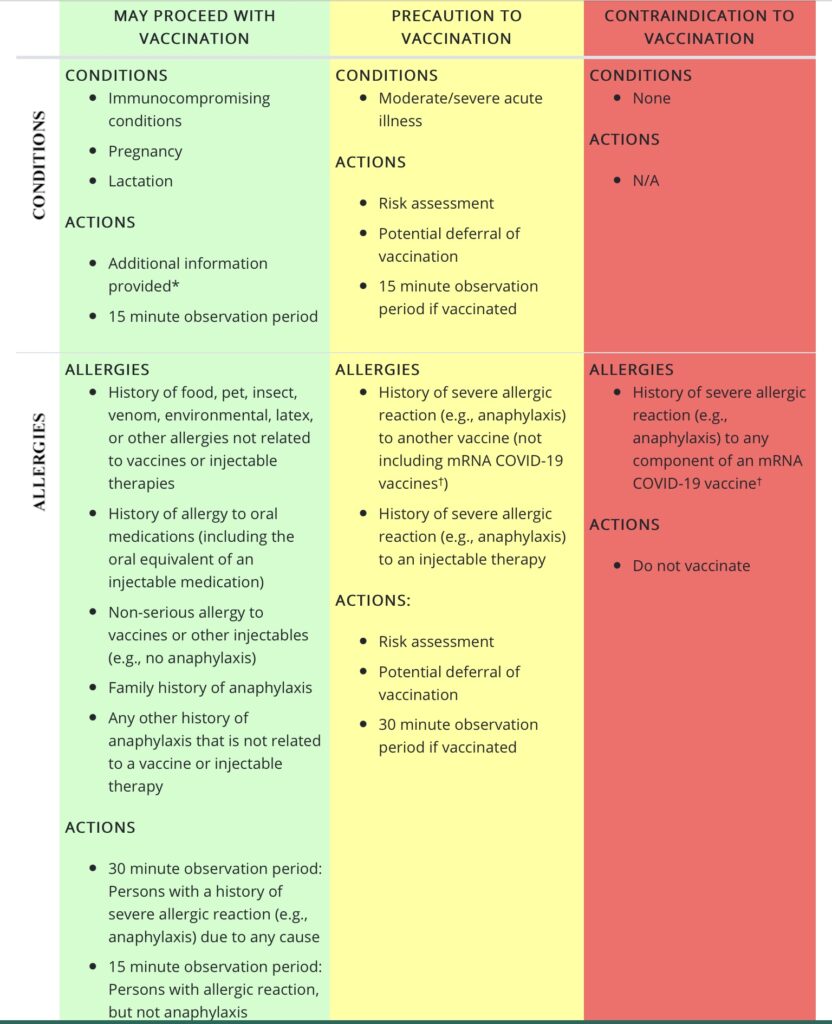
* See special populations section on CDC website for information on patient counseling in these groups. Photo credit cdc.gov.
2. Bell’s Palsy
Bell’s Palsy is a condition that affects the 7th cranial nerve, causing facial droop on the affected side. The cause is not entirely known but viral and/or immune triggers are suspected. It is most often treatable, with return to normal function. The National Organization for Rare Disorders (NORD) lists the rate of Bell’s Palsy in the general U.S. population as 25-35 out of every 100,000 persons (if we take the average, that is 0.03% of the population affected).
In the Pfizer study, there were 4 cases of Bell’s Palsy in the vaccine group and none in the placebo group. While the numerical imbalance is certainly noteworthy and needs further observation as we roll these vaccines out to the general public, let’s put these cases in perspective. The incidence of Bell’s Palsy in the vaccine arm of the Pfizer trial was 0.02% (4 out of 18,555 participants who received both doses of vaccine) – LOWER than the general population rate.
In the Moderna study, there were 3 cases of Bell’s Palsy in the vaccine group and one case in the placebo group. That puts the incidence in the vaccine group also at 0.02% (3 out of 13,982 participants who received both doses of vaccine) – again, LOWER than the background rate of disease.
3. Lymphadenopathy
Lymphadenopathy refers to the enlargement or swelling of lymph nodes. Lymph nodes are part of our immune system. They filter out damaged cells and produce and store our lymphocytes (white blood cells), which fight infection. We notice our lymph nodes most often when experiencing illness. Strep throat, for example, causes significant swelling of the lymph nodes in the front of the neck. We have lymph nodes all over our bodies and these will often react to sources of infection or inflammation.
In both the Pfizer and Moderna trials, lymphadenopathy was noted in a certain percentage of vaccine recipients. This was most often noted under the arm in which the injection was given. While this doesn’t happen super commonly with vaccines, it does happen. And it shouldn’t surprise us. As we discussed in Part 1 of this series, immunizations trigger our immune system. In so doing, our lymph nodes enlarge as they get activated and kick into gear.
Lymphadenopathy experienced by study participants typically resolved within several days.
Are vaccine manufacturers really “liability free”?

The story of liability regarding vaccines is not as straightforward as some would have you believe. There are avenues for recourse in the case of harm from vaccines. Photo credit Pixabay.
Not exactly. Here’s a bit of vaccine history to help clarify. In the 1980s, lawsuits against vaccine manufacturers threatened to put some out of business and to cause vaccine shortages. This put the country at risk of decreased immunization rates and a return of deadly vaccine preventable diseases. “In recognizing that there are known, but rare, serious side effects from vaccination and that people who suffer those consequences deserve to be compensated, the federal government set up the NVICP” (National Vaccine Injury Compensation Program). This served to “reduce the burden on vaccine manufacturers and to provide restitution to those seriously affected by vaccine adverse reactions.” (Let’s Talk Vaccines: A Clinician’s Guide to Addressing Vaccine Hesitancy and Saving Lives, Wolters Kluwer Press, 2019)
The NVICP allows people to be compensated on a “no fault” basis. This means that they don’t have to prove fault on the part of a manufacturer or medical provider to receive compensation. As long as the reaction is acknowledged as likely caused by the vaccine, examples of which are listed in the Vaccine Injury Table (VIT), claimants are eligible for compensation. And if a reaction is not on the table, the claimant just had to show a plausible theory backed by a credible expert.
Not all vaccines are included in the VIT, however. So, if a person has a reaction they feel is related to a non-included vaccine, they can seek compensation through the traditional court system. Also, if claimants are denied compensation through the NVICP, they can then seek suit against a manufacturer or clinician through the courts. In this situation, the claimant would have to prove fault or negligence on the part of the manufacturer or clinician.
The NVICP does NOT prevent liability of vaccine manufacturers in the case of vaccine defects. As an example, let’s say a vaccine manufacturer failed to fully inactivate a virus before it was marketed and administered to the public as a “killed virus vaccine”. If people contracted the disease from the vaccine, that manufacturer would then be liable. But they are not held liable for things that we KNOW are inherent risks in vaccinating – passing out, allergic reactions, etc. If someone has a severe enough reaction to warrant compensation, the government (through the NVICP) program, foots the bill.
Just so you don’t worry that this is likely to happen, the FDA inspects manufacturing plants and production processes and it tests vaccine lots before they go out to the public. These measures help ensure that something like the scenario listed above is less likely to happen.
The important footnote to all of this is that there is a parallel plan in place for products listed as “countermeasures” employed during a pandemic or biologic attack. This program, the Countermeasures Injury Compensation Program (CICP), is much more stringent and, honestly, difficult to navigate and gain compensation from. Read here and here for a good discussion of the benefits and limitations of both programs.
Pfizer vs Moderna – mostly, it’s a draw

These vaccines are so similar that, at an individual level, they both are excellent choices. Photo credit Pixabay.
The Pfizer and Moderna vaccines are both mRNA vaccines. They have the same mechanism of action. Please read here for a more detailed discussion of how these vaccines work. Vaccine availability is limited enough that we really won’t have a choice about which vaccine we’ll have access to – at least, not right away. And really, these vaccines are similar enough that we can feel confident getting either one. Regardless, let’s do a side-by-side comparison of these vaccines looking at some of the more important characteristics to consider.
Well, I had a really lovely table prepared but it didn’t quite transfer over as I’d hoped. So, here is my best attempt. Thanks for bearing with me!
Vaccine info: Pfizer/BioNTech Moderna
Age 16+ 18+
Dose 30 mcg /0.3 ml 100 mcg/0.5 ml
Packaging 5-6 doses/vial 10 doses/vial
Dosing Interval 21 days 28 days
Storage -70 degrees C -20 degrees C
Administration Intramuscular Intramuscular
Preparation Thaw and dilute Thaw
Preservative None None
Participants ~44,000 ~30,000
Countries US/Argentina/Brazil/S.Africa US only
Diversity (vaccine/placebo)
Amer. Indian or 0.7%/0.6% 0.8%/0.8%
Alaskan Native
Asian 4.4%/4.4% 4.4%/4.9 %
Black/African American 9.8%/9.7% 9.8%/9.6%
Native Hawaiian or 0.3%/0.1% 0.2%/0.2%
Pacif. Islander
White 81.8%/82.1% 79.5%/79.3%
Hispanic or Latino 26.3%/26.1% 20%/19.9%
65+ yr old 21.6% 25.3%
Female 49.4% 47.4%
High risk conditions 46.2% 22.3%
Efficacy 95% overall 94.1% overall
Safety No significant safety signals noted in either trial
Ingredients
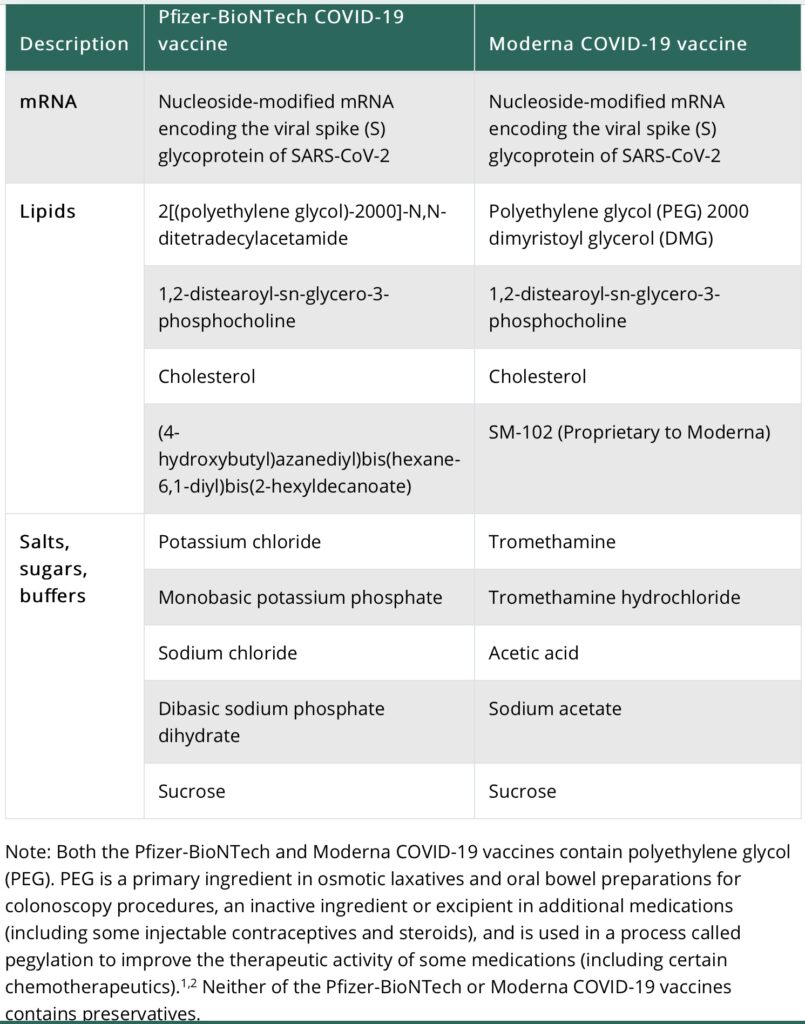
Head to head comparison of vaccine ingredients for the Pfizer and Moderna COVID vaccines. Photo credit cdc.gov.
And hot off the presses…
The Advisory Committee on Immunization practices voted this weekend to adjust the phases of vaccine distribution. Here is the latest.

Well, folks. It’s been a pleasure. I will sign off for now but am sure that I’ll be updating you soon as more things change (and they are certain to change).
If you want more vaccine-related info, don’t forget to subscribe to this blog. You can find the subscribe button on the home page. Until next time…


Thank you for this excellent information!
Amy Cross, Public Health Nurse @ SRHD
Thank you so much, Amy!