Breaking News – COVID vaccines for kids 6 months to 5 years are finally here! We now have two vaccines (Moderna and Pfizer) authorized for emergency use by the FDA and CDC to protect our youngest children from COVID. This is an exciting time for parents who have put life on hold to protect their young children who were previously ineligible for COVID vaccination. And it is an exciting time for pediatricians and family physicians who have been anxiously waiting to protect our youngest patients.
We now have nearly 2 years of experience with COVID vaccination in adults and teens. And we have a tremendous amount of data (more than for practically any other vaccine we offer) showing these vaccines to be extremely safe and effective in preventing severe outcomes of infection. Vaccinating our youngest children protects their health and futures, releases their families from the bonds of worry, and brings us one step closer to ending this pandemic.
In just a bit, we’ll take a deeper dive into these vaccines, as parents will likely have a choice between the two. BUT, before we get to that, let’s review some important general concepts about COVID vaccines in kids. These will hopefully help address common questions and provide a framework with which to think about the need for COVID immunization in this age group. Also, please note the vocabulary section below. Vaccine trials use some terms (noted in italics) that need explanation, and I will do my best to explain them in a way that is clear and easy-to-understand.
COVID vaccination in children – general concepts:
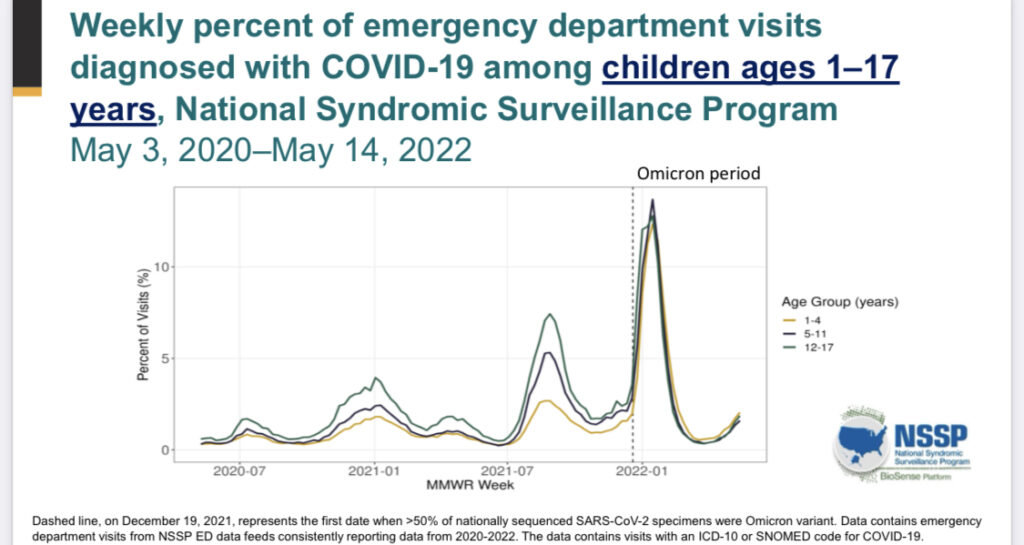
- COVID illness in children is common, particularly more so since Omicron became the dominant strain (Image A).
- Serious outcomes of COVID illness in children are more common during the Omicron period (Image B).
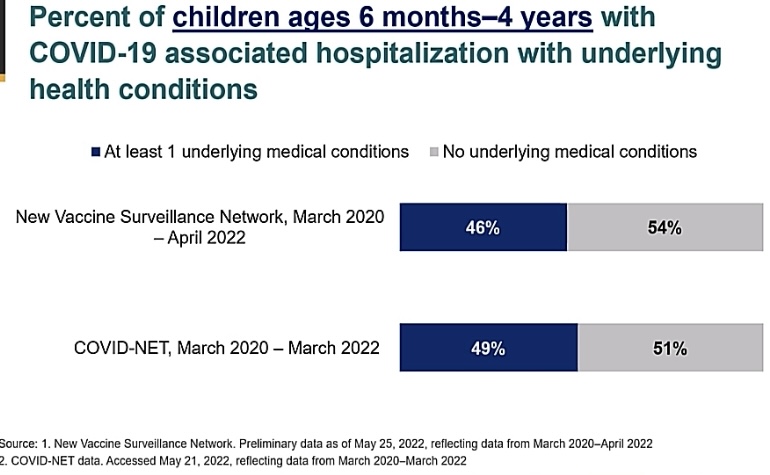
- Serious outcomes are not only a concern for the medically fragile child. More than 50% of kids with serious outcomes had no risk factors for severe disease (Image C).
- Having had COVID illness previously does not necessarily protect from repeat illness with a different strain (we now have 5 different sub-variants of Omicron and people previously infected with an earlier sub-variant are now getting sick with later sub-variants).
- Prior mild illness with COVID does not necessarily predict mild illness with future infections.
- We should think of vaccination against COVID in much the same way we think about Influenza vaccination.
- Variants are emerging rapidly and repeat vaccination will be necessary
- The primary goal of vaccinating is to decrease the severity of disease (severity of symptoms, hospitalization, cases of MIS-C, and death)
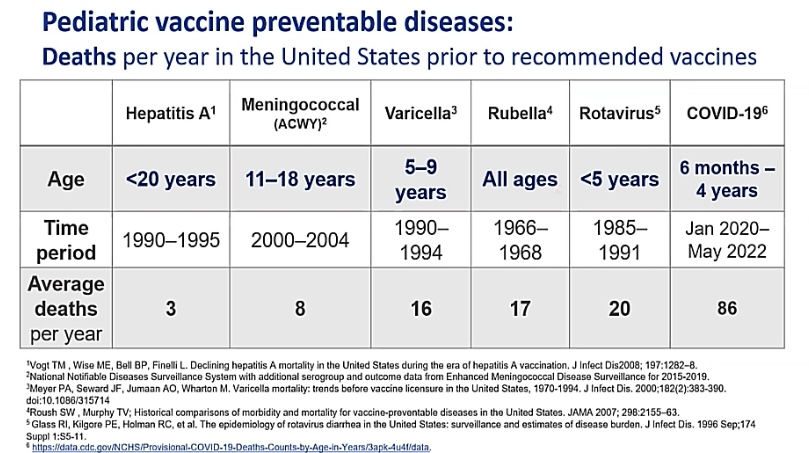
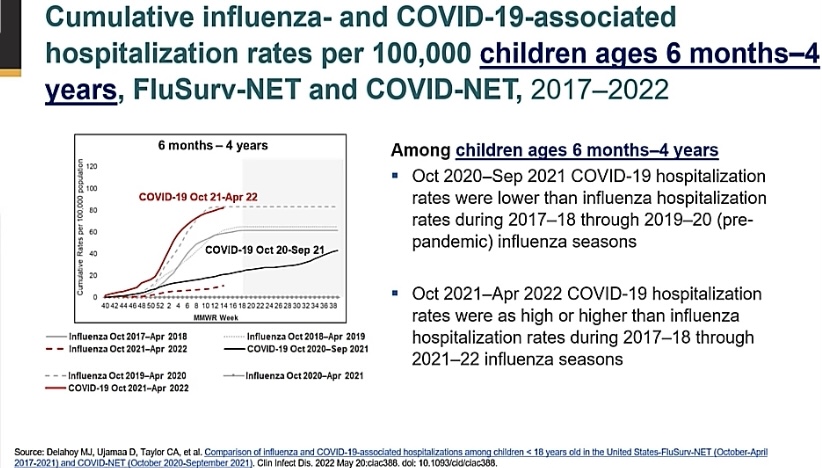
- Illness and serious outcomes are more common from COVID19 than from other vaccine-preventable diseases for which we already vaccinate (Image D and Image E).
- There has been more vaccine safety monitoring for COVID19 vaccines than for any other vaccines we have ever had, and the safety profile of these vaccines is excellent!
- Rates and types of reactogenicity (fatigue, headache, fever, lymphadenopathy, etc.) after vaccination in kids was similar to, or less common than, in teens and adults.
- COVID19 vaccines have now been administered to more people in the US and worldwide than any other vaccine we’ve ever had. They have proven exceedingly safe in real world use.
- COVID isn’t just affecting the health of the individual. Children absolutely play a role in transmission of this virus. Vaccinating our children will help decrease transmission to others, allowing them to protect their families and communities.
- Preventing COVID illness is not just about preserving physical health. The COVID pandemic has had huge negative effects on the social and emotional wellbeing of our children. There are higher rates of depression and anxiety. General academic performance has suffered. And social interactions so vital to childhood development have been severely limited. We vaccinate not only to protect health but to allow our kids to get back to the business of being kids.
Image A: Demonstrates rise in cases in children of all ages during the Omicron period. Slide from ACIP June 2022 presentation.
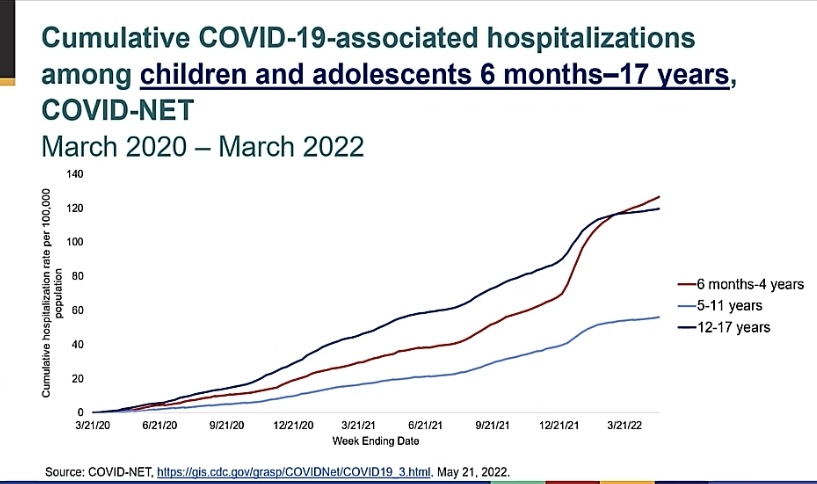
Image B: Increase in COVID-19 associated hospitalizations noted most prominently during the Omicron period (late 12/2021 to present). Slide from ACIP June 2022 presentation.
Image C: Demonstrating that a larger percentage of children 6 months to 4 years hospitalized with COVID-19 had no risk factors for serious disease. Slide from ACIP June 2022 presentation.
Image D: Table showing deaths per year in the US prior to recommended vaccines. Deaths from COVID are significantly greater than deaths from other vaccine-preventable diseases. Slide from ACIP June 2022 presentation.
Image E: Figure showing that rates of serious COVID disease (as measured by hospitalizations) is as high or higher during the Omicron period than rates of influenza hospitalizations in the 2019-20 flu season. Slide from ACIP June 2022 presentation.
A deeper dive
Let’s compare the data on the Moderna and Pfizer vaccines. Both have proven extremely safe in pediatric trials, though each has its benefits and limitations. No matter which you have available or which you choose, I hope you’ll come away with the big picture message that any COVID vaccine (Moderna or Pfizer) is better than no vaccine at all!

Slide comparing trial data on Moderna and Pfizer vaccines for kids 6 months to 5 years.
†The interval between dose 2 and 3 in the trial was longer than the authorized interval, ranging from 8-32 weeks in the 6-23 months age group and between 8-34 weeks in the 2-4 years age group.
*For both vaccines, efficacy endpoints are in participants with and without evidence of prior infection, while immunogenicity data is only in participants without evidence of prior infection.
**There is lower confidence in Pfizer efficacy estimates due to only 3 COVID cases in the vaccine group and 7 cases in the placebo group. There is greater confidence in Moderna efficacy estimates based on 181 COVID cases in the vaccine group and 97 cases in the placebo group. NOTE: this does not mean that there were more cases of illness in the vaccine group than the placebo group. Remember, there were 3 times as many participants who received vaccine than received placebo – 3.7% of vaccine recipients became infected vs 6.1% of those receiving placebo.
††SAE = Serious Adverse Event. In the Pfizer trial, there were more SAEs in the placebo group than in the vaccine group. Numbers of events in both trials were very low, but the number of trial participants was smaller. However, given our experience with older children and adults, we don’t expect to see worrisome numbers of SAEs as larger numbers of children are vaccinated. There were NO cases of vaccine-related anaphylaxis, myocarditis/pericarditis, or death in the trials.
***Due to Pfizer’s extension of trial when the initial 2 dose series failed to provide protection, a certain number of participants were unblinded and offered vaccination after 6 months (hence, the longer intervals in some participants between doses 2 and 3). Only 32% of the eligible study population contributed blinded person-time to the efficacy evaluation.
Let’s discuss…
There are a variety of things we need to keep in mind as we look at this data. Beyond what was explained above, remember that these trials were conducted primarily during the Omicron period of the COVID pandemic. The data on adults and teens was collected during earlier variant waves and cannot be directly compared. To compare effectiveness of pediatric vaccines to teen and adult vaccines, we must look at the effectiveness of teen and adult vaccines during the same Omicron period. In this light, the effectiveness is similar.
The overall lower effectiveness against symptomatic disease, compared to earlier variant waves, is not a commentary on the vaccine itself. Rather, it reflects the viral mutations that have occurred, rendering the virus better able to evade vaccine and infection-induced immunity. In adults and children, however, COVID vaccines are still highly effective in protection against serious outcomes of disease (see below). This is what we should be focusing on!
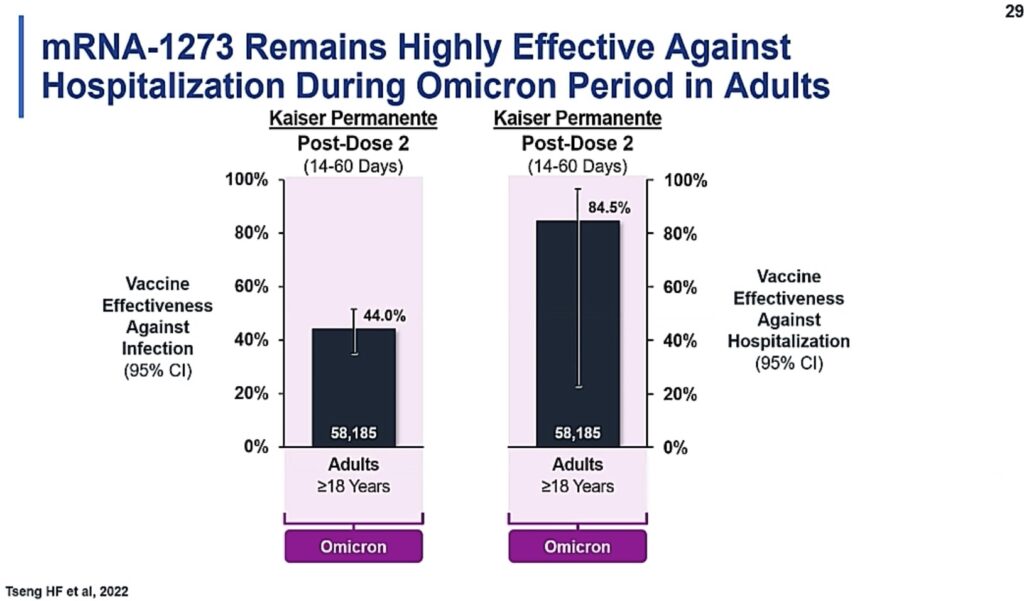
Slide from ACIP June 2022 meeting showing continued effectiveness against severe outcomes of disease (such as hospitalization) during the Omicron period.
As we look to roll out these vaccines, and as parents consider which vaccine to give their child, we must remember that the Pfizer vaccine is a 3 DOSE primary series. The effectiveness after 2 doses is minimal (this is why researches extended the trial to include a third dose). Children must receive all 3 doses to be protected. Parents should not have false hope that two doses would provide any measure of protection.
And while Moderna’s vaccine has greater efficacy after 2 doses, the protection offered is moderate. It is highly likely that Moderna will recommend a third dose (or “booster” dose) in the future. On the plus side, that moderate protection is afforded more quickly with the Moderna vaccine series than with the Pfizer vaccine series, which takes a longer time to complete.
Even though dosing of the Moderna and Pfizer vaccines is quite different (25 mcg vs 3 mcg, respectively), the tolerability profile is similar. The higher dose doesn’t necessarily mean more reactogenicity.
Summing up
In summary, both vaccines are safe, and both are effective at preventing severe outcomes of disease. As time goes on and as we learn more about the virus and the body’s response to the vaccines, we should expect that scientists and public health experts will adjust how these vaccines are given. They may recommend a different interval between vaccine doses for maximal protection. They may change the composition of the vaccine to improve coverage of different strains of virus. But while we are learning and improving, I hope you will choose to vaccinate your child. Let’s protect them now so that they may remain healthy long enough to see the development of more effective vaccines in the future.
And for you teens and adults, if you have received your primary series of vaccines but are more than 3-6 months out from the second dose, the data suggests that protection against Omicron is negligible (see below). To remain protected, you MUST get that booster dose. If you or your child is eligible for a booster and haven’t yet gotten it, please rush right out and do so!
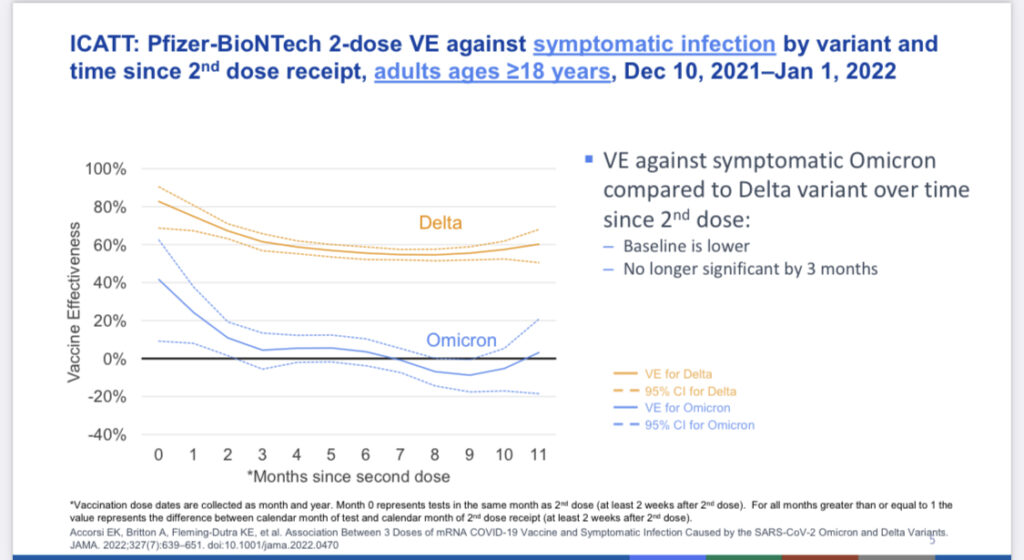
Slide from ACIP June 2022 meeting demonstrating severely reduced effectiveness of 2-dose series of COVID vaccines against symptomatic infection with the Omicron variant by 3-6 months after the 2nd dose.
Well, that’s it for now folks. I hope this has been helpful. Thanks for tuning in and feel free to drop any further questions in the chat! If you’d like further vaccine updates delivered to your inbox, hop on over to the home page and subscribe.
And for you clinicians out there who are preparing to vaccinate your youngest patients, you can find some helpful information on the various vaccination schedules (they differ slightly for immune competent and immune suppressed children) here.
Vocabulary:
- Immuno-bridging: We can tell a vaccine’s effectiveness in 2 ways. First, we look at how much it prevents disease in those vaccinated compared to those unvaccinated. This looks at the whole person level. Second, we can look at effectiveness by examining how many antibodies are in a person’s blood or how good those antibodies are at neutralizing/fighting virus in a lab environment. This looks at vaccine response at a more microscopic level. Immuno-bridging studies are controlled trials, using placebo or other controls and correlates of protection (see definition below) to establish whether an intervention is effective. In the pediatric trials, scientists then compare the results from the pediatric group to that from teens and adults (where we have much more data) to see if the data is “non-inferior”, meaning effectiveness was similar to, or better than, that seen in the older age groups.
- Correlates of protection: correlates of protection are “measurements of immune parameters that allow prediction of the degree of protection against infection or disease induced by a pathogen. A study of antibody levels in more than 12,000 health care workers exposed to SARS-CoV-2 indicated that serum antibody levels to either the spike or nucleocapsid proteins correlated with protection against infection.” In COVID studies, we therefore use antibody levels as correlates of protection.
- Vaccine efficacy/effectiveness (VE): While vaccine efficacy and effectiveness are slightly different, I will define them similarly. Vaccine efficacy/effectiveness is interpreted as the reduction in disease among a vaccinated group compared to an unvaccinated group. A VE of 90% indicates a 90% reduction in disease occurrence among the vaccinated group, or a 90% reduction from the number of cases you would expect if they have not been vaccinated.
- Reactogenicity: These are symptoms that can occur following vaccination. These include things like muscle pains, fatigue, injection site swelling, fever, lymph node swelling, headache, nausea, vomiting, diarrhea, dizziness, etc. These are unpleasant, to be sure. But these symptoms are actually signs that our immune system is doing its job. An immune response is an inflammatory response, and these are signs of inflammation in the body. Thankfully, these are typically short-lived and mild and MUCH less severe than the reaction to a real infection.
- Unblinded: Blinding means that the vaccine trial participant doesn’t know whether they are getting a vaccine or placebo. Double-blinded trials mean that neither the participant nor the researcher knows who’s getting a vaccine or placebo. In the case of the Pfizer trial, after 6 months (when those later enrolled in the study of 3 doses had received the third dose and significant protection from vaccination was noted), those children in the placebo part of the trial were unblinded. This means that the parents of kids in the placebo arm were given the opportunity to get a vaccine for their child. This is done for ethical reasons. Knowing that the intervention (vaccination) is effective at preventing illness, it would be ethically questionable to allow children to remain unprotected and at risk when a successful intervention is available to them.